Degree of Dissociation Formula
Conceptual ideas develop logically and sequentially ultimately leading into the mathematics of the topics. Thioldisulfide exchange is the principal reaction by which disulfide bonds are.

Calculate The Degree Of Dissociation Of Hi At 450 C If The Equilibrium Constant For The Diss Youtube
Degree of dissociation of an equilibrium involving gas can be calculated by knowing the equilibrium constant and the concentration of the gaseous reactantproduct.
. Hence thioldisulfide exchange is inhibited at low pH typically below 8 where the protonated thiol form is favored relative to the deprotonated thiolate form. This calculator converts automatically the pressure to bar with the following conversion factor. Hence you should be sure of the fact that our online essay help cannot harm your academic life.
In aqueous solution the equilibrium of acid dissociation can be written symbolically as. The obtained osmotic pressure with formula 2 is in psi pounds per square inch. Systematic studies of plasma began with the research of Irving Langmuir and his colleagues in the 1920s.
Pure sulfuric acid does not exist. For example the proximity or contact with graphite during heating will cause rapid dissociation of the SiO2. Here we will study the pH value formula and how pH value is calculated in detail.
Type 2 diabetes mellitus is a chronic metabolic disorder associated with hyperglycaemia caused by impaired insulin secretion and insulin resistance. The pK a of a typical thiol group is roughly 83 but can vary due to its environment. Enzymes are proteins that speed up chemical reactions necessary for life in which substrate molecules are converted into products.
For example in the decomposition of carbonate the number of moles of carbon dioxide can be calculated from the equilibrium constant by assuming an ideal gas behavior. I There will be more undissociated ammonia molecules than undissociated methylamine molecules. Divided by the number of formula units initially dissolved in solution and means the number of particles per formula unit of the solute when a solution is dilute.
Get 247 customer support help when you place a homework help service order with us. Acid strength is the tendency of an acid symbolised by the chemical formula to dissociate into a proton and an anion The dissociation of a strong acid in solution is effectively complete except in its most concentrated solutions. A pH of more than 7 is classified as basic.
Must contain at least 4 different symbols. The following expression is used to mathematically represent molar conductivity. An enzyme inhibitor is a molecule that binds to an enzyme and blocks its activity.
With course help online you pay for academic writing help and we give you a legal service. At 25C using solutions of the same concentration for example 01 mol L-1. The molar conductivity of an electrolyte solution is defined as its conductivity divided by its molar concentration.
The degree of dissociation is the fraction of the original solute molecules that have dissociated. This service is similar to paying a tutor to help improve your skills. Sulfuric acid American spelling and the preferred IUPAC name or sulphuric acid Commonwealth spelling known in antiquity as oil of vitriol is a mineral acid composed of the elements sulfur oxygen and hydrogen with the molecular formula H 2 SO 4It is a colorless odorless and viscous liquid that is miscible with water.
From the Editor. Each lesson includes informative graphics occasional animations and videos and Check Your Understanding sections that allow the user to practice what is. An enzyme facilitates a specific chemical reaction by binding the substrate to its active site a specialized area on the enzyme that accelerates the most difficult.
In the case of specific conductivity it is seen that the conductivity increases as the concentration of. Langmuir also introduced the term plasma. For dissociation in the absence of association the van t Hoff factor is.
We will guide you on how to place your essay help proofreading and editing your draft fixing the grammar spelling or formatting of your paper easily and cheaply. The haze can be removed from the surface by a gentle heating in the oxy-hydrogen flame. Where HA is an acid that dissociates into A- known as the.
κ is the measured conductivity formerly known as specific conductance c is the molar concentration of the electrolyte. Methanol Type of Substance Composition. A pH of 7 is regarded as neutral.
Thiolates not thiols attack disulfide bonds. Examples of strong acids are hydrochloric acid perchloric acid nitric acid and sulfuric acid. The SI unit of molar conductivity is siemens metres squared per mole S m 2 mol 1.
Colligative constants in degrees Celciusm Kf value of water. Dear Readers Contributors Editorial Board Editorial staff and Publishing team members. The pH scale which normally spans from 0 to 14 in water is used to determine the pH of an aqueous solution.
Acidity is defined as a pH of less than 7. 6 to 30 characters long. Formula for degree of unsaturation is given below.
If you do not have a water analysis you can use the values given in the right column in the input table. 82 - 100 ww Constituent 1. ASCII characters only characters found on a standard US keyboard.
K b ammonia 18 10-5 smaller. Plasma was first identified in laboratory by Sir William CrookesCrookes presented a lecture on what he called radiant matter to the British Association for the Advancement of Science in Sheffield on Friday 22 August 1879. HA H 2 O A-H 3 O.
An acid dissociation constant K a is a quantitative measure of the strength of an acid in solutionIt is the equilibrium constant for a chemical reaction known as dissociation of acidbase reactions. Under typical atmospheric conditions water vapor is continuously generated by. In genetics dominance is the phenomenon of one variant of a gene on a chromosome masking or overriding the effect of a different variant of the same gene on the other copy of the chromosome.
The first variant is termed dominant and the second recessiveThis state of having two different variants of the same gene on each chromosome is originally caused by a mutation in one of. Stepping Down When I became editor-in-chief of The American Journal of Cardiology in June 1982 I certainly did not expect to still be in that position in June 2022 forty years laterMore. An official publication of the American Academy of Allergy Asthma and Immunology The Journal of Allergy and Clinical Immunology brings timely clinical papers instructive case reports and detailed examinations of state-of-the-art equipment and techniques to clinical allergists immunologists dermatologists internists and other physicians concerned.
From the Editor in Chief interim Subhash Banerjee MD. However values are often quoted in S cm 2. Conversely such electrolytes have lower molar conductivity at higher concentrations due to a reduced degree of dissociation.
Water vapor water vapour or aqueous vapor is the gaseous phase of waterIt is one state of water within the hydrosphereWater vapor can be produced from the evaporation or boiling of liquid water or from the sublimation of iceWater vapor is transparent like most constituents of the atmosphere. In this Primer DeFronzo et al. A weak acid is only partially dissociated with.
The Physics Classroom Tutorial presents physics concepts and principles in an easy-to-understand language. The dissociation is greatly enhanced when the heating of fused quartz is carried out in reducing conditions. K b methanamine 44 10-4 larger.
Our online services is trustworthy and it cares about your learning and your degree. Assuming 100 dissociation calculate the freezing point Tf and boiling point Tb of 327 m K3PO4aq. 1 psi 6894810-2 bar.
Compare the values of the base dissociation constant for ammonia and methylamine.

Calculate The Percentage Degree Of Dissociation Of An Electrolyte Xy 2 Normal Molar M Youtube
Degree Of Dissociation Pka Of Weak Acid Calistry
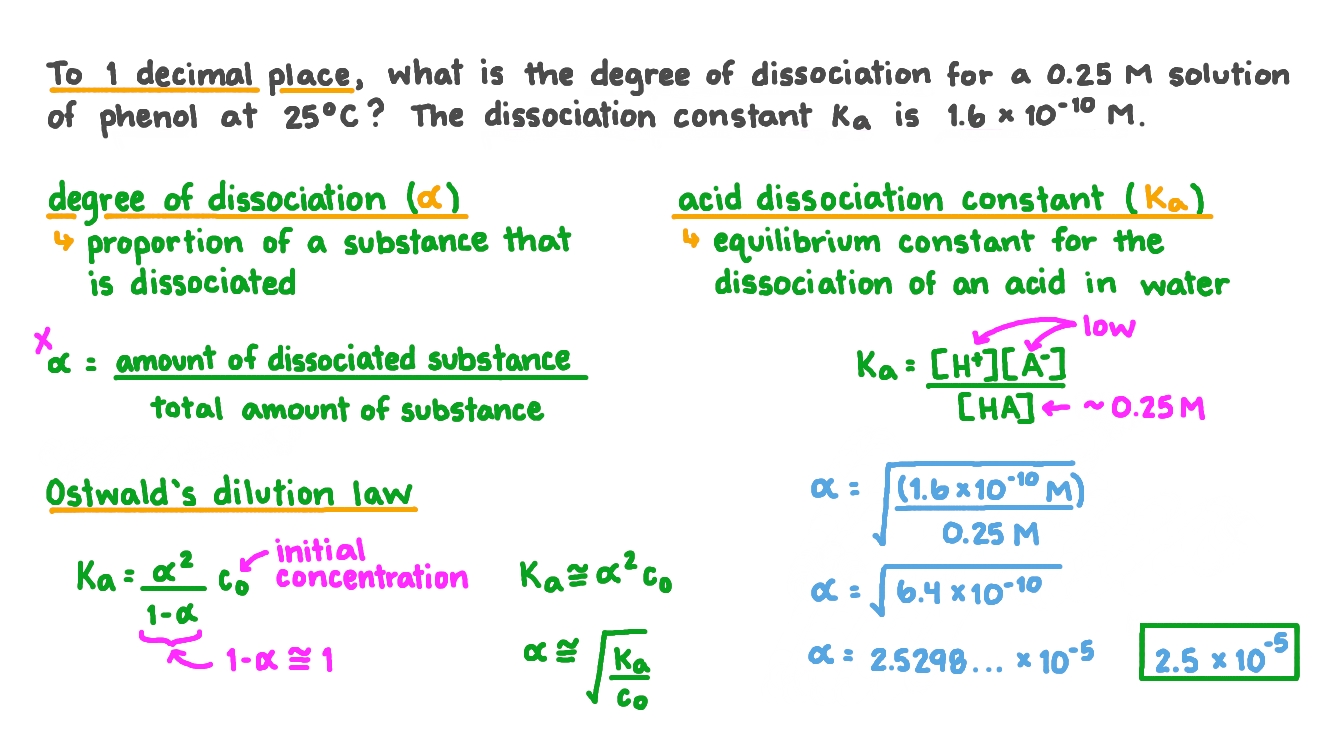
Question Video Calculating The Degree Of Dissociation Of A Solution Of Phenol Given The Acid Dissociation Constant Nagwa
If The Degree Of Dissociation Is Given Then How To Take The Values At Equilibrium Q 2hi H2 I2 The Degree Of Dissociation Is A Calculate Expression For Equilibrium Constant Kc Of The
No comments for "Degree of Dissociation Formula"
Post a Comment